H2so4 Naoh Balanced Equation
The balanced equation will have six atoms of oxygen on each side of the equation. Ii PbO C Pb CO2.

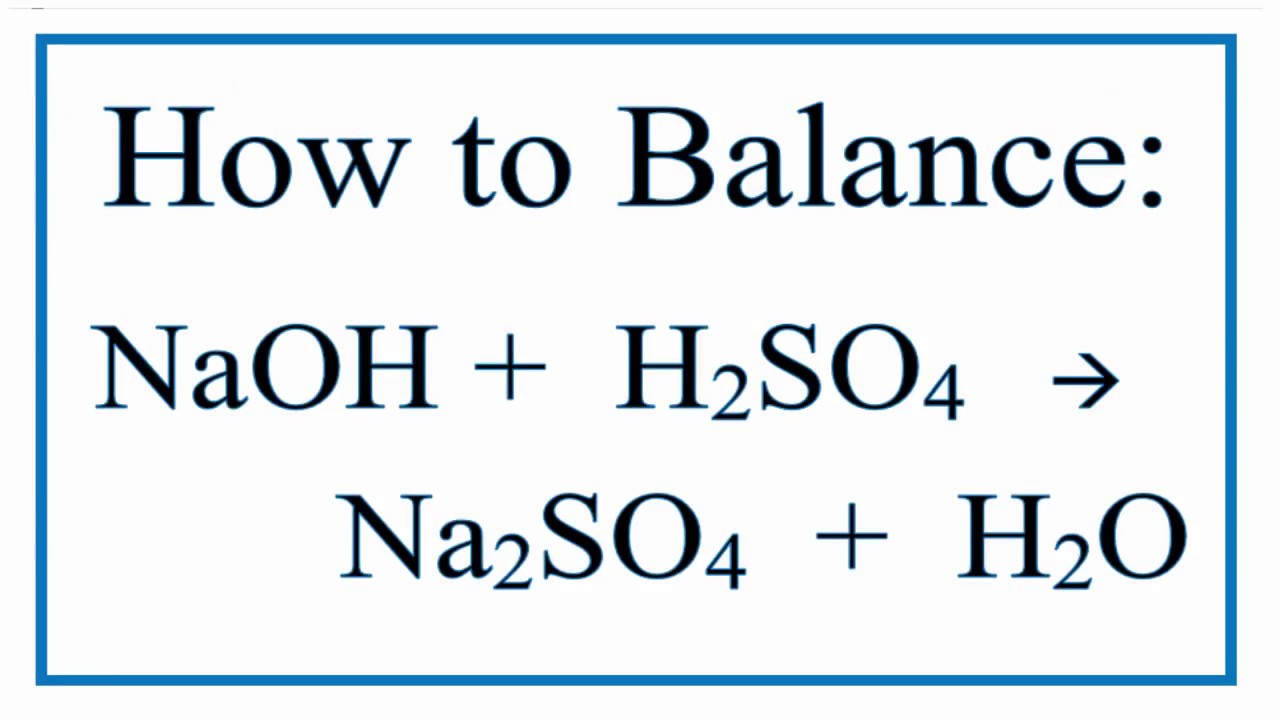
Type Of Reaction For H2so4 Naoh Na2so4 H2o Youtube
Balance the equation NaOH H2SO4 Na2SO4 H2O using the algebraic method.
. The equivalence point is where the moles of titrant and. How many motes of Fe2 ion can he oxidized by 13 times 10-2 moles MnO_4-. Fe Au Co Br C O N F.
What is the formula mass in atomic mass units for H2SO4. Once you know how many of each type. For 20 ml acid solution.
Order and degree of a differential equation formulation of a differential equation whole general solution is given variables separable method. CBSE 2012 i Reddish brown metal is copper. Add drops of acid to water carefully with constant stirring as the reaction is exothermic.
Lets say a molecule HNO3 is having a normality of 05. Determine a balanced equation for the reaction of molecular nitrogen N2 and oxygen O2 to form dinitrogen pentoxide. Calculate the concentration of a 25 mL NaOH solution if 35 mL of 125 M HCl is needed to titrate to the equivalence point.
Water forms a compromise between H2O and H3O and -OH. Harris QCA 8e Experiments. Balance the chemical equation.
Ii Write balanced chemical equation for both the equation. A chemical equation can be balanced as follows by using algebraic method. Physical SciencesGrade 11 9 DBE2015 Examination Guidelines NOTE.
In this equation the mole ratio of NaOH base and HCl acid is 11 as determined by the balanced chemical equation. Therefore x 9 10-3 equivalent because it is a monobasic acid the mass of the titration equation of the acid. So the number of base equivalents 12 15 18 10-3 equivalent.
The balanced complete ionic equation for the reaction when aqueous NiCl2 and aqueous question_answer Q. Use uppercase for the first character in the element and lowercase for the second character. Water H2O can act as.
Write the balanced net ionic equation for the reaction between MnO_4- ion and Fe2 ion in acid solution. For NaOH valency is one as it gives one OH- ion when it dissolves in water. Fe Au Co Br C O N F.
When it is heated in china dish presence of oxygen black coloured copper oxidised is formed. KClO3s KCls O2g is not balanced. For Sulphuric acid H2SO4 the valency is 2 as you get 2 H ions when you dissolve it in water.
If there is more than one object a free-body diagram must be drawn for each object and Newtons second law must be applied to each object separately. I NaOH H2So4 Na2So4 H2O. The reaction was performed using 45 g NaHCO3 and 18 g NaOH were produced.
Sulphuric acid can not be neutralised by water but can be diluted with water. Download Free PDF Download PDF Download Free PDF View PDF. Titaration of Vinegar with NaOH.
When an object accelerates the equation Fnet ma must be applied separately in the x and y directions. However the most common clinical. Use uppercase for the first character in the element and lowercase for the second character.
Label Each Compound With a Variable. Download Free PDF Download PDF Download Free PDF View PDF. P4O10 H2O -- H3PO4 What is the numerical coefficient in A.
The balanced equation will have one atom of chlorine on each side of the equation. Is sulfuric acid ionic or molecular. To neutralise sulphuric acid strong bases like NaOH can be used.
The balanced equation will appear above. Ordinary Differential Equations. The titration calculations for NaOH.
Balance the following chemical equation. To balance NaOH HCl NaCl H2O youll need to be sure to count all of atoms on each side of the chemical equation. So in 20 ml of acidic solution 180 x 10-3 equivalent of acids.
15 ml 012 mol NaOH required. The balanced equation will appear above. For HCl Hydrochloric acid valency is one because when you dissolve it in the water you get one H ion.
Ii 2Cu O₂ 2CuO. Label each compound reactant or product in the. Ionic charges are not yet supported and will be ignored.
Equation of a plane distance of a point from a plane condition for coplanarity of three lines angles between two planes angle between a line and a plane. Balancing of the equation means that Number of reactant side atom. To measure the amount of chlorine in a well-boring fluid an analytical chemist adds 03100M silver.
By Nur Shahida Abu Hanifah. HCl NaOH NaCl HOH H2SO4 2 NH4OH NH42SO4 2 HOH 2 NaOH H2CO3 N2CO3 2 NaOH. Balance this equation then select the statement that best describes the balanced equation.

Sodium Hydroxide And Sulfuric Acid Yields Sodium Sulfate And Water Youtube

Naoh H2so4 Na2so4 H2o Chemical Equation Balancer

Sodium Hydroxide Sulfuric Acid Acid Base Neutralization Reaction Youtube
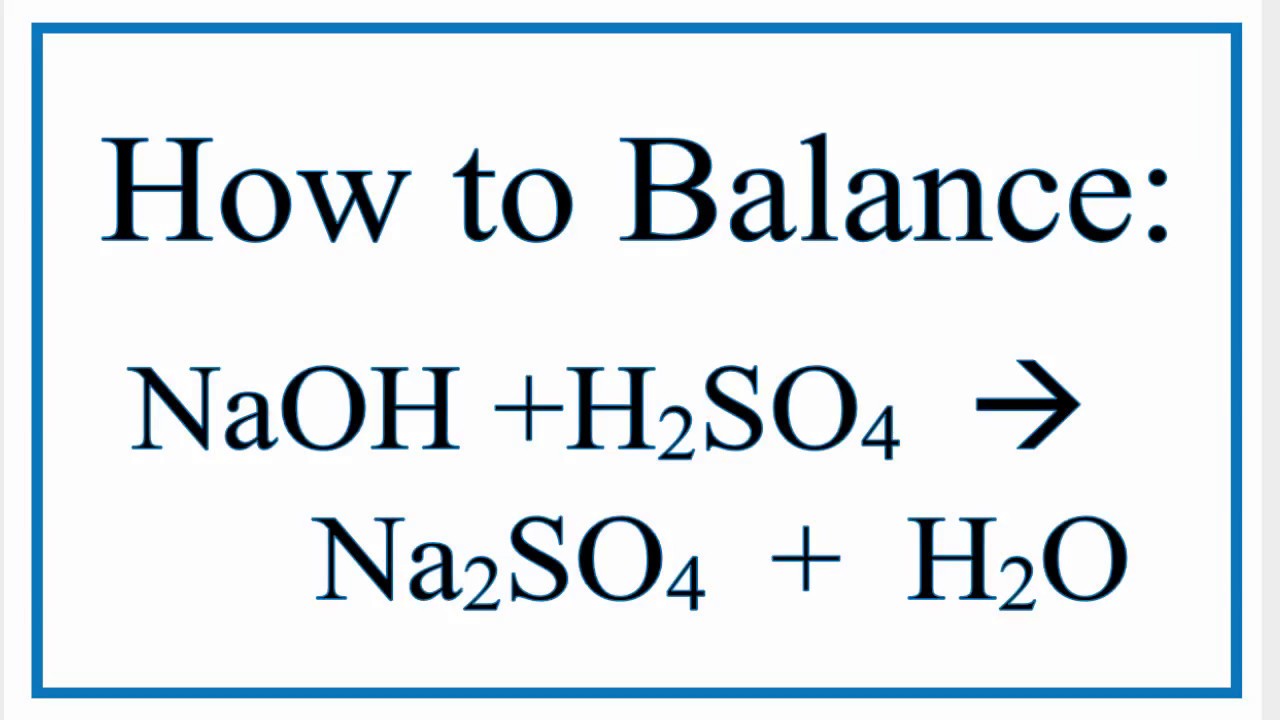
How To Balance Naoh H2so4 Na2so4 H2o Youtube
0 Response to "H2so4 Naoh Balanced Equation"
Post a Comment